
We know lithium batteries mainly from our smartphones, laptops, or smartwatches: all devices that can be recharged again and again once the battery is empty, but not all lithium batteries are rechargeable. Some chemistries, generally CR (lithium manganese dioxide) and ER (lithium thionyl chloride) batteries, may not be recharged under any circumstances. Both types of batteries are available as button and round cells. CR and ER batteries are used in many applications in homes, including, for example, electronic gas or electricity meters. Nowadays, they are also increasingly being used in the automotive sector. Due to their relatively low power consumption and long-term operation, they do not need to be changed too often.

How is the service life determined?
The life of non-rechargeable lithium batteries can be determined from the currents drawn and the ambient temperatures in which the battery is used. The long service life of CR and ER batteries (several years) can withstand a wide range of temperatures due to the low self-discharge of the battery chemistries. However, there are also limitations, especially with lithium thionyl chloride batteries:
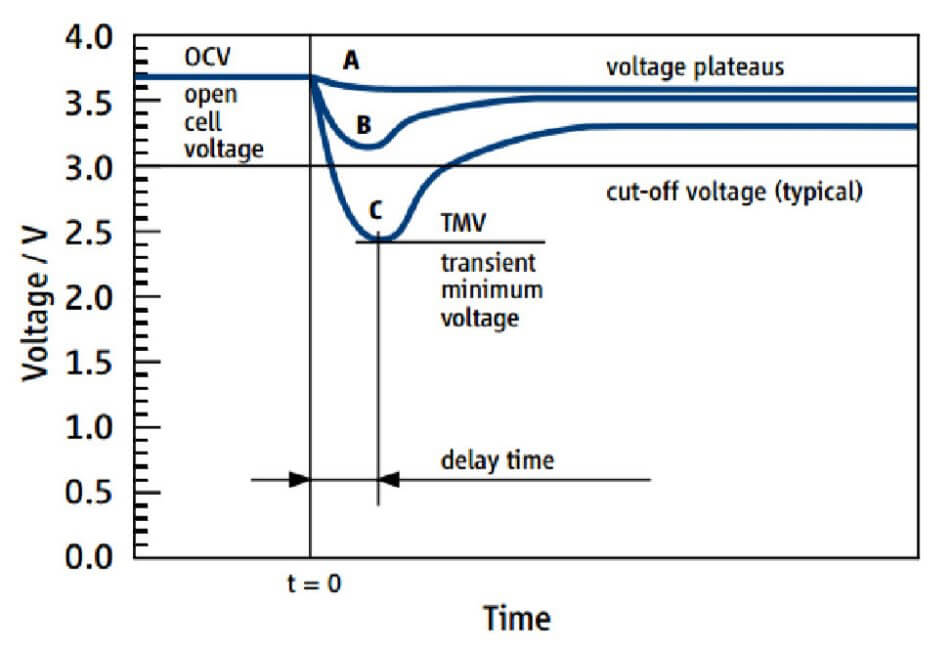
Lithium manganese dioxide batteries have a lower voltage (3.0 volts) than lithium thionyl chloride batteries (3.6 volts) and do not show significant voltage drops during storage or when loaded with extremely small loads. This is not the case for lithium thionyl chloride batteries. These batteries have a voltage drop at the beginning of use due to a passive layer that forms on the lithium during non-use, which must first be “broken up”. Depending on the storage time and the ambient temperature of the battery, this leads to a drop in voltage, also known as voltage delay. It should be noted that the voltage delay can be critical after a long period of time and where the device has a relatively high minimum voltage. This simulates a short battery life.
Do you need a (primary) battery for your applications? Our experts will be happy to advise you on different options for your individual project.


 Deutsch
Deutsch 



